Some of the pictures on this website come from the Internet. If you think we infringe your copyright, please let us know and delete them immediately
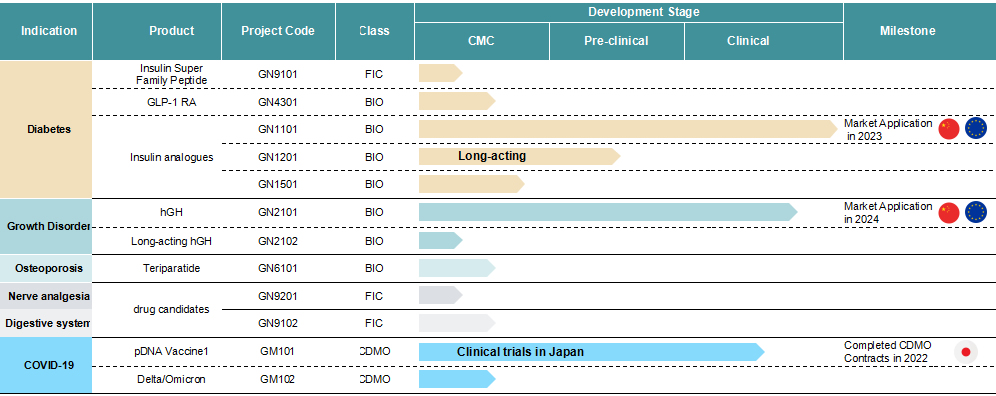
10+ product pipeline addressing US$100 billion market potential in the coming decades
Focus on chronic endocrine and metabolic diseases such as diabetes, strive to provide high-quality long-term affordable solutions
Two EU clinical late-stage biosimilar assets. Since China joined ICH in 2017, the regulatory policies and registration requirements have been updated to adopt FDA/EMA’s guidance and review standards, supporting Genova to advance co-registration in both EU and China
The EU clinical trial of one insulin analog is expected to be completed in Q4 2022. The mid-stage EU clinical trial of recombinant hGH is expected to be completed in Q4 2022 while the late-stage in 2023
A First-in-Class drug candidate with unique mechanism of action; a co-authored manuscript was submitted to Nature Chemical Biology for publication, targeting to treat diseases with unmet clinical needs
Established platform technology for nucleic acid vaccine manufacture. Completed CDMO service contract orders for a Covid-19 vaccine candidate for a Japan-based client